TrialPoint™
TrialPoint™ is a Research Management System (RMS) that facilitates the collection of, and rapid access to, research data for clinical trials across the globe. The system was conceived and developed from the ground up by the team at Databean to enable us to manage more research with fewer resources.
TrialPoint™ EDC
TrialPoint™ electronic data capture (EDC) is easy to configure, easy to use, and the coordinators love it. Our data managers are experts in converting clinical protocols into geek speak (metadata dictionaries for those in the know) and will configure TrialPoint™ to work for you, not against you. The system can accept any randomization string, media upload, or coding dictionary. It can even blind users to treatment assignments. To promote time-sensitive data collection. the system will prospectively calculate visit windows and send out alerts and reminders keeping the team focused and on task.


TrialPoint™ ePRO
Nowadays, patient-reported health outcomes and quality of life questionnaires are included in most trial designs and are responsible for many protocol compliance issues. Take a look at TrialPoint’s electronic patient-reported outcomes (ePRO) module which can be configured to fit any quality of life questionnaire or patient diary. This secure patient portal can be accessed remotely (including on a mobile device), can prompt patients to enter information within a defined window, and can automatically score the instruments providing you with analysis-ready data.
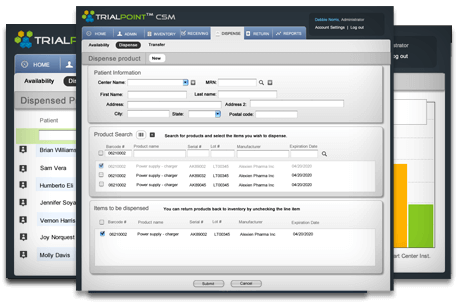
TrialPoint™ CSM
Often, the most time-consuming and frustrating part of clinical research is keeping track of investigational products. With TrialPoint™ clinical supply management (CSM), you can record the shipping, use, and return of drugs, devices, and clinical supplies. This module integrates seamlessly with the EDC system to provide comprehensive real-time reporting on all inventories — which makes it ideal for single product companies constantly shifting low inventory.